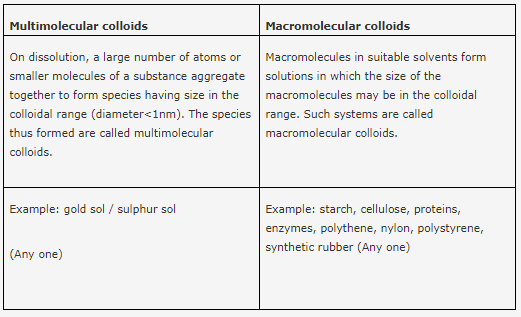
What is the difference between multimolecular and macromolecular colloids? Give one example of each type. How are associated colloids different from these two types of colloids?
Some substances at low concentrations behave as normal strong electrolytes, but at higher concentrations exhibit colloidal behaviour due to the formation of aggregates. The aggregated particles thus formed are called associated colloids or micelles. The formation of micelles takes place only above a particular temperature called Kraft temperature and above a particular concentration called critical micelle concentration. On dilution, these colloids revert back to individual ions.