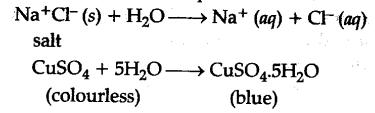Interaction of H+ ions and OH" ions of ${{H}_{2}}$0 with anions and cations of the salt respectively to give an acidic or a basic solutoin is called hydrolysis. For example:
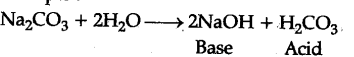
Hydration, on other hand, means addition of water to ions or molecules to form hydrated ions or hy¬drated salts. For examples: