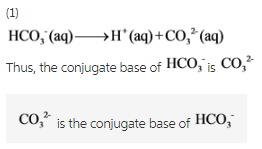1)What is the conjugate base of HCO3−?
Express your answer as a chemical formula.
2)What is the conjugate acid of HPO32− ?
Express your answer as a chemical formula.
3)Among three bases, X−, Y−, and Z−, the strongest one is Y−, and the weakest one is Z−. Rank their conjugate acids, HX, HY, and HZ, in order of decreasing strength.
Rank the acids from strongest to weakest. To rank items as equivalent, overlap them.
Concepts and reason
Conjugated acid-base theory was explained by Lowry-Bronsted acid base.theory. This theory states that acid is a substance that donates a proton and base is a substance that accepts a proton.
Fundamentals
Conjugate acid: is species that formed by the acceptance of a proton.
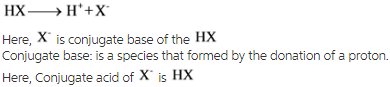
The conjugate acid of a strong base will be weak and that of a weak base will be strong.
Answer: