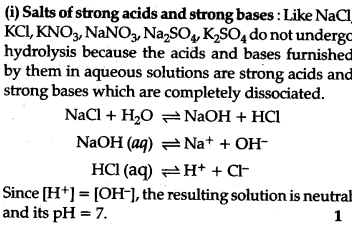What is salt hydrolysis ? Explain hydrolysis of salts of:
(i) strong adds and strong bases,
(ii) strong adds and weak bases,
(iii) strong bases and weak adds,
(iv) weak adds and weak bases.
Salt hydrolysis: Hydrolysis is a process in which a salt reacts with water to form add and base.
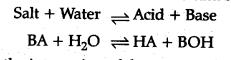
This is the interaction of the cations of the salt with ions furnished by water and anions of the salt and H+ ions furnished by water to form an acidic or basic solution is called salt hydrolysis.