what is redox reaction? give an example.
Oxidation and Reduction: Redox Reactions
Oxidation and reduction reactions occur simultaneously. When oxidation occurs in one substance, reduction takes place in the other substance. Such reactions are called redox reactions. In the name ‘redox’, the term ‘red’ stands for reduction and ‘ox’ stands for oxidation.
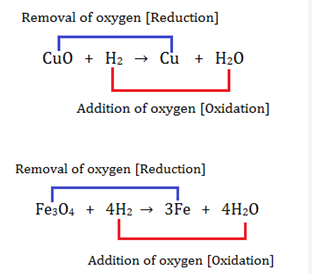
In both, the above reactions, oxidation and reduction occur simultaneously. One of the reactants acts as an oxidising agent and gets reduced, while the other acts as a reducing agent and gets oxidised.