What is ‘Plaster of Paris’ chemically? How is it obtained from gypsum? Write the condition and chemical equation involved in its manufacture?
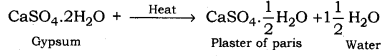
Plaster of Paris is calcium sulphate hemihydrate (CaSO4. 1/2H2O).
Gypsum, when heated at a temperature of about 380 K in a kiln, loses its three-fourth water of crystallisation and changes to plaster of Paris.
Following is the reaction involved
 :
: