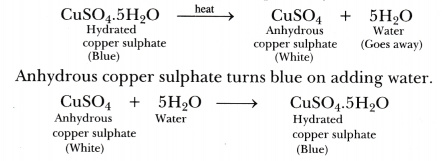
What is meant by water of crystallisation? Explain that the crystalline salts contain water of crystallisation.
Answer:
Water of crystallisation is a fixed number of water molecules present in one formula unit of a salt. One formula unit of copper sulphate contains five water molecules (5H20). The water molecules which form part of the structure of a crystal are called water of crystallisation. When hydrated salts are heated strongly, they lose their water of crystallisation.
On strong heating, blue copper sulphate crystals turn white (due to the loss of water of crystallisation).