(i) Delocalisation: Delocalisation means that pairs of 71 electrons extend over 3 or more atoms. They belong to the whole molecule. For example, 6n electrons present in benzene are delocalised and are spread on the whole of the ring and this imparts extra stability to the molecule.
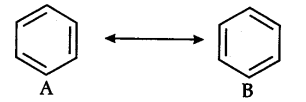
(ii) Resonance energy: The difference between the energy of the most stable contributing/canonical structure and the energy of the resonance hybrid is known as resonance energy. In case of benzene, the resonance hybrid as 147 kj {{mol}^{-1}} than either A or B below. Thus resonance energy of benzene is 147 kj {{mol}^{-1}}.
2 Likes
So mam , can it all be explained like this also
The energy difference between actual energy and expected energy of a molecule. ?
1 Like