what is difference between tetrahidal and octahidral voids on the basis of there shapes.?
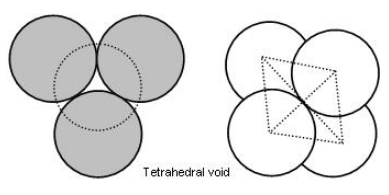
Tetrahedral Voids
In close packing arrangement, each sphere in the second layer rests on the hollow (triangular void) in three touching spheres in the first layer. The centers of these four spheres are at the corners of a regular tetrahedral. The vacant space between these four touching spheres is called tetrahedral void.
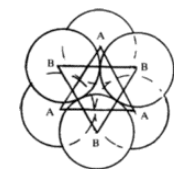
Octahedral void
In the same packing one half of the triangular voids in the first layer are occupied by spheres in the second layer while the other half remains unoccupied. The triangular voids ‘b’ in the first layer is overlapped by the triangular voids in the second layer. The interstitial void, formed by combination of two triangular voids of the first and second layer is called octahedral void because this is enclosed between six spheres centres of which occupy corners of a regular octahedron.
The difference:
In tetrahedral void s- In a close packing, the number of the tetrahedral void is double the number of spheres, so there are two tetrahedral voids for each sphere.
In a multi-layered close-packed structure, there is a tetrahedral hole above and below each atom hence there is twice as many tetrahedral holes as there are in close-packed atoms
Radius of the tetrahedral void relative to the radius of the sphere is 0.225.
In octahedral Voids - In close packing, the number of octahedral voids is equal to the number of spheres. Thus, there is only one octahedral void associated with each sphere. Radius of the octahedral void in relation to the radius of the sphere is 0.414.