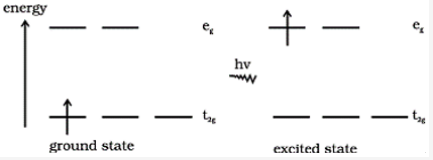
What is d-d transition?
The d orbitals in the transition elements do not have the same energy in their complexes. It is a transition where an electron jumps from one d orbital to another. Normally these are degenerate i.e., the d orbitals have the same energy, but under some conditions, such as the presence of ligands, the degeneracy can be removed so that there is a specific energy and therefore wavelength associated with these transitions.
These sorts of transitions sometimes have energies located in the visible band, and it’s one reason transition metal ions and complex ions in particular tend to be highly colored.