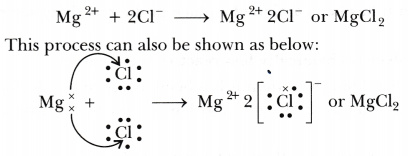- What is an ionic bond?
- How is an ionic bond formed?
- Write the formation of magnesium chloride.
Answer:
- The chemical bond formed by the transfer of electrons from one atom to another is known as an ionic bond.
- An ionic bond is formed when one of the atoms can donate electrons to achieve the inert gas electronic configuration and other atom needs electrons to achieve the inert gas electronic configuration.
When a metal (usually 1, 2 or 3 electrons in outermost shell) reacts with a non-metal (usually 5, 6 or 7 electrons in outermost shell), transfer of electrons takes place from metal atoms to the non-metal atoms and an ionic bond is formed. There is a strong force of electrostatic attraction between metallic cation and non-metallic anion which is responsible for the formation of ionic bond. -
Formation of magnesium chloride (MgCl2): The atomic number of magnesium is 12. It has two electrons in its valence shell as shown below:
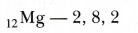
Magnesium, therefore, has a tendency to lose the 2 valence electrons and in the process attains the electronic configuration of neon.
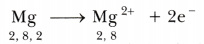
Chlorine (atomic number 17) has 7 electrons in the valence shell. It has a tendency to gain one electron to complete its octet.
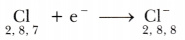
Thus, when magnesium and chlorine are brought together, the magnesium atom transfers its two valence electrons to two chlorine atoms. In the process, both the atoms acquire the stable electronic configuration of nearest inert gases. The positively charged magnesium ion Mg2+ and negatively charged chloride ions (Cl-) are now held together by the electrostatic force of attraction and form ionic bond.