Consider the reactions,
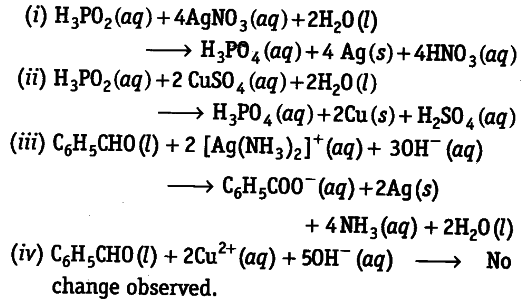
What inference do you draw about the behaviour of {{Ag}^{+}} and ${{Ag}^{2+}}$from these reactions?
In reactions, (i) and (ii) reactions, ${{AgNO}{3}}$ and
${{CusO}{4}}$ act as oxidising agents respectively. They oxidise
$H_{2}PO_{2}$(hypophosphorous acid) to (orthophosphoric acid). In reaction (tit),
[Ag(NH3 )2]+(aq) oxidises benzaldehyde to benzoic acid but in reaction (iv), ${{Cu}^{2+}}$ do not oxidise benzaldehyde to benzoic acid.
This indicates that Ag+ is a stronger oxidising agent than ${{Cu}^{2+}}$.