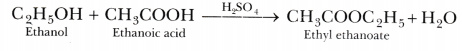What happens when
- ethanol burns in air.
- ethanol reacts with sodium metal.
- ethanol is oxidised with chromic anhydride in glacial ethanoic acid.
- ethanol is heated with alkaline potassium permanganate.
- ethanol is heated with ethanoic acid in the presence of a few drops of concentrated sulphuric acid?
Answer:
- Ethanol is highly inflammable liquid. It catches fire easily and starts burning. Ethanol burns readily in air with a blue flame to form carbon dioxide and water vapour:
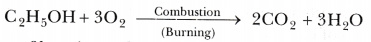
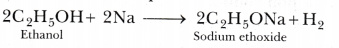
A lot of heat is produced during the combustion of ethanol. - Ethanol reacts with sodium to produce sodium ethoxide and hydrogen gas:
- When ethanol is treated with chromic anhydride, then its partial oxidation takes place and ethanal is formed.

Chromic anhydride oxidises ethanol to ethanal. - Alkaline KMn04 oxidises ethanol to ethanoic acid.
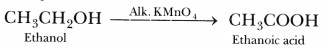
- Ethanol reacts with ethanoic acid in presence of concentrated sulphuric acid to form a sweet smelling ester, ethyl ethanoate.