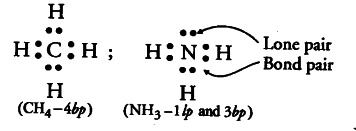What do you understand by bond pairs and lone pairs of electrons? Illustrate by giving one example of each type.
Covalent bond is formed by mutual sharing of electrons. The shared pair of electrons present between the bonded atoms are called bond pairs of electrons and unshared pair (non-bonding) electrons are called the lone pairs of electrons, e.g., ammonia, { NH }_{ 3 } contains 3 bond pairs and 1 lone pair of electrons, whereas
{ CH }_{ 4 } contains only 4 bond pairs.