What are the steps involved in the contact process of manufacture of sulphuric acid.
Steps Involved in the Contact process
-
Production of Sulphur Dioxide
SO2 is produced by roasting metallic sulphides in air.
4FeS2 +11O2 → 2Fe2O3+ 8SO2 -
Purification of Gases
To enhance the efficiency of a catalyst, various impurities present in the mixture of sulphur dioxide and air are first removed. -
Catalytic Oxidation of Sulphur Dioxide
Oxidation of SO2 to SO3at 450°C in the presence of catalyst vanadium pentaoxide.
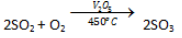
-
Absorption of Sulphur Trioxide in Sulphuric Acid
Sulphur trioxide vapours are absorbed by a stream of conc. sulphuric acid.
SO3 + H2SO4 → H2S2O7 (oleum or pyrosulphuric acid) -
Dilution of Oleum to Obtain Sulphuric Acid
A calculated amount of water is added to obtain sulphuric acid of desired strength.
H2S2O7 + H2O → 2H2SO4