(i) What are the reactions involved in removing ${{SO}_{2}}$ from the atmosphere by passing it through a solution containing citrate ions?.
(ii) What is the most important sink of CO pollutant?
(iii) How are flue gases from industries freed from oxides of nitrogen and sulphur?
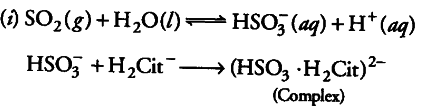
(ii) Soil microorganisms
(iii) By scrubbing them with cone. H_{2}SO_{4} or with alkaline solutions like Ca${{(OH)}{2}} and
Mg{{(OH)}{2}}$.