What are amphoteric oxides? Choose the amphoteric oxides from amongst the following oxides:
Na2O, ZnO, Al2O3 CO2, H2O.
(a) Metal oxides that react with both acids and bases to produce salt and water are known as Amphoteric oxides. For example, zinc reacts with an acid or a base to produce salt and water.
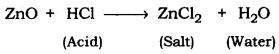
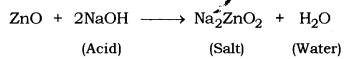
Amongst the given oxides, zinc oxide (ZnO) and aluminium oxide (Al2O3) are amphoteric oxides.
(b) Non-metals are electron acceptors. Hence, they cannot donate electrons to H+ ions to form hydrogen gas (H2).