(i) Justify the given statement with suitable examples “the properties of the elements are a periodic function of their atomic numbers”.
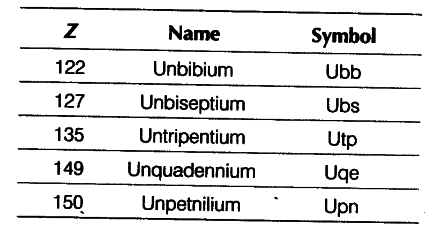
(ii) What would be IUPAC names and symbols for elements with atomic numbers 122, 127, 135, 149 and 150?
(i) There are numerous physical properties of elements such as melting points, boiling points, heats of fusion and vaporisation, energy of atomisation, etc., which show periodic variations. The cause of periodicity in properties is the repetition of similar outer electronic configuration after certain regular intervals, e.g., all the elements of Is group (alkali metals) have similar outer electronic configuration, i.e.,{{ns}^{1}}.
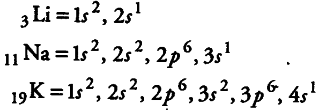
Therefore, due to similar outermost shell electronic configuration all alkali metals have similar properties. e.g., sodium and potassium both are soft and reactivemetals. They all form basic oxides and their basic character increases down the group. They all form unipositive ion by the lose of one electron. Similarly, all the elements of 17 th group (halogens) have similar outermost shell electronic configuration, i.e., {{ns}^{2}} {{np}^{5}} and thus possess similar properties.
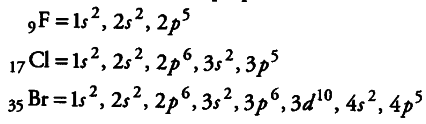
(ii) The roots 2, 7, 5, 9 and 0 are referred as bi, sept, pent, enn and nil, respectively. Therefore, their names and symbol are