Using the given data, calculate the rate constant of this reaction. **
** A+B ----> C+D
Trial A(M) B(M) Rate(M/s)
1. 0.340 0.200 0.0142
2. 0.340 0.520 0.0960
3. 0.476 0.200 0.0199
k =_____ ?
Answer:
Calculate order of reaction as follows:

Compare powers,
Value of n is 2.
Now, calculate order with respect to reactant A
 Compare powers therefore, m is equal to 1.
Compare powers therefore, m is equal to 1.
For calculating the rate constant, order of reactants is needed. Order is calculated by using rate equation.
Calculate rate constant as follows:
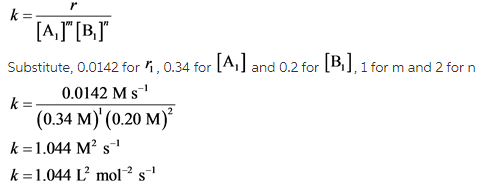
![]()
Rate constant is calculated by using rate equation. For calculating rate constant, order of reactants is calculated.
![]()