Using electron transfer concept, identify the oxidant and reductant in the following redox reactions.
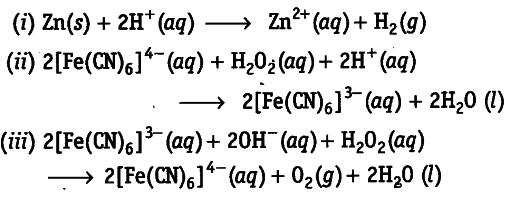
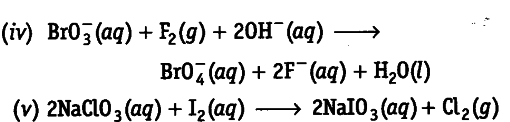
Oxidants (i) ${{H}^{+}}$ (ii)$H_{2}O_{2}$ (iii) [Fe(CN)6]3-
(iv) $F_{ 2 }$ (vi) NaCl${{O}{3}}$
Reductants (i) Zn (it) [Fe(CN)6]4~
(iii) $H{2}O_{2}$ (iv) Br${{O}{3-}}$
(v) 2$I{ 2 }$