The variation of vapour pressure of different liquids with temperature is shown in figure below,
(i) Calculate graphically boiling point of liquids A and B.
(ii) If we take liquid C in a closed vessel and heat it continuously, at what temperature will it boil?
(iii) At high altitude, atmospheric pressure is low (say 60 mm Hg). At what temperature will liquid D boil?
(iv) Pressure cooker is used for cooking food at hill station. Explain in terms of vapour
pressure Why is it SO?
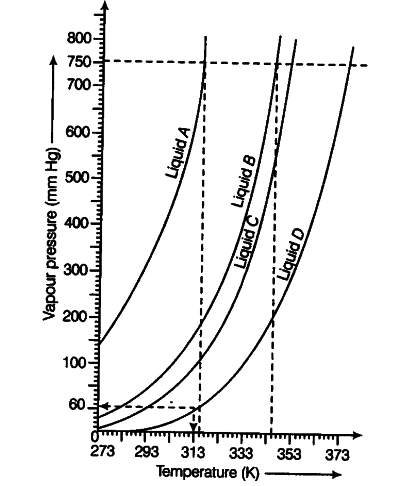
(i) Boiling point of liquid A— 315 K, B — 345 K.
(ii) In a closed vessel, liquid C will not boil because pressure inside keeps on increasing.
(ii) Temperature corresponding to 60 mm =313 K.
(iv) A liquid boils when vapour pressure becomes equal to atmospheric pressure. At hill station, atmospheric pressure is low. Therefore, liquid boils at a lower temperature and cooking is not perfect. In a pressure cooker, the pressure inside the cooker increases and the liquid boils at a higher temperature.
