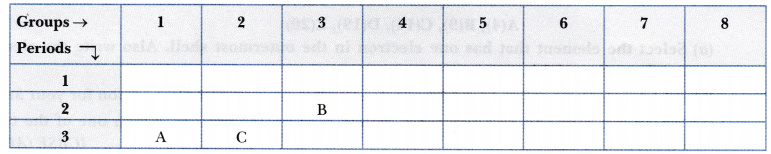
The position of three elements A, B and C in the Periodic Table is shown below:
Giving reasons, explain the following:
- Element A is a metal.
- Element C has larger size than element B.
- Element B has a valency of 3.
Answer:
- Element A belongs to group 1 of the Periodic Table. So, it has 1 valence electron and it forms unipositive ion by losing its valence electron. Hence, A is a metal.
- This is because element C is in third period while element B is in second period. We know that the atomic size increases down the group and decreases along a period.
- Element B is in 2nd period and 3rd group hence it has 3 valence electrons. It loses 3 electrons to achieve nearest inert gas configuration. So, its valency is 3.