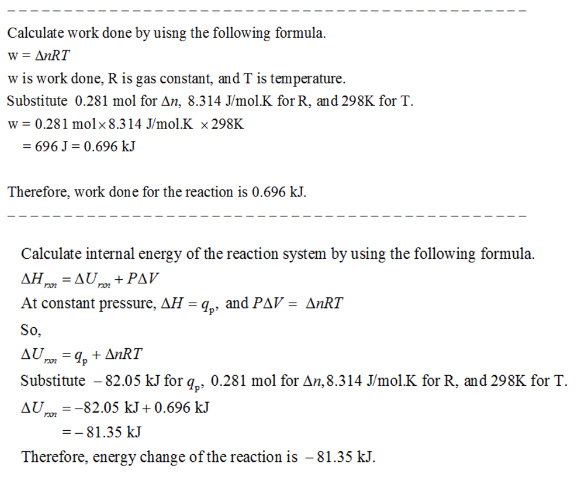
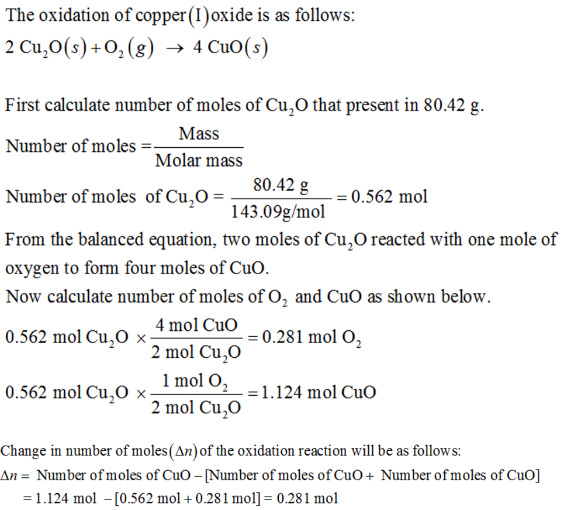
The oxidation of copper(I) oxide, Cu2O(s), to copper(II) oxide, CuO(s), is an exothermic process.
The change in enthalpy upon reaction of 80.42 g of Cu2O(s) is -82.05 kJ.
Calculate the work, w, and energy change, ΔUrxn, when 80.42 g of Cu2O(s) is oxidized at a constant pressure of 1.00 bar and a constant temperature of 25 °C.
Answer: