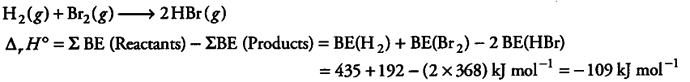The net enthalpy change of a reaction is the energy required to break all the bonds in reactant molecules minus amount of energy required to form all the bonds in the product molecules. What will be the enthalpy change for the following reaction
${ H }{ 2 }$ +
${ Br }{ 2 }$ ----------> 2HBr
Given that bond energy of ${ H }{ 2 }$,
${ Br }{ 2 }$ and HBr is 435 kJ /mol,192 kJ /mol and 368 kJ /mol respectively.