The Ksp of PbBr2 is 6.60*10^-6 ?
- What is the molar solubility(M) of PbBr2 in pure water?
- What is the molar solubility(M) of PbBr2 in 0.500M KBr solution?
- What is the molar solubility(M) of PbBr2 in a 0.500M Pb(NO3)2 solution?
Concepts and reason
Solubility:
Solubility is defined as the maximum quantity of solute dissolved in a given amount of solvent to make a saturated solution at a particular temperature.
Molar solubility:
Molar solubility is the number of moles of a solute that can be dissolved in one liter of a solution. It is expressed as mol/L or M (molarity).
Common ion effect:
Common ion effect is defined as the solubility of a partially soluble salt which will decrease with the addition of a soluble salt that has an ion in common with it.
Fundamentals
Consider a general reaction:
![]()
The relation between solubility product and molar solubility is as follows:
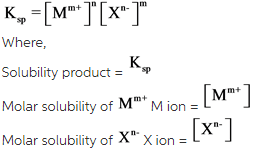
Answer:
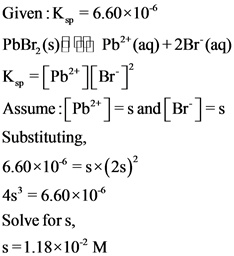
Therefore, the molar solubility (M) of ![]() in pure water is
in pure water is ![]() .
.
Explanation
The given compound is slightly soluble in water and the solubility product constant is ![]() . The molar solubility (M) of
. The molar solubility (M) of ![]() in pure water is calculated using the solubility product expression.
in pure water is calculated using the solubility product expression.
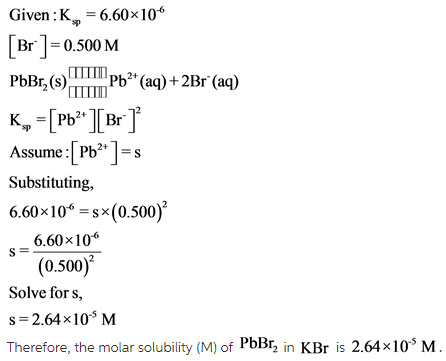
Explanation
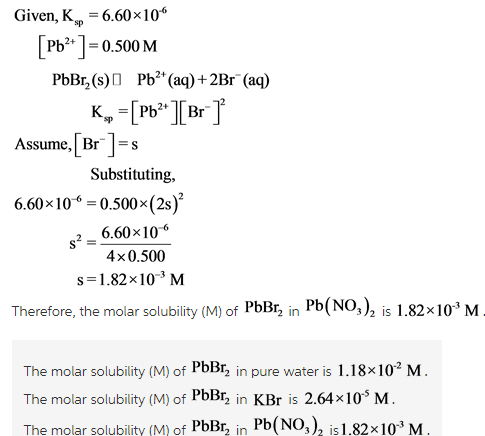
Explanation

