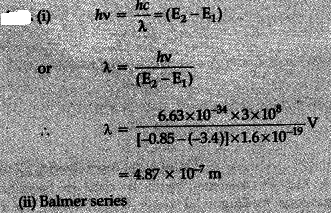The ground state energy of hydrogen atom is - 13.6 eV. If an electron makes a transition from an energy level - 0.85 eV to - 3.4 eV, calculate the wavelength of the spectral line emitted. To which series of hydrogen spectrum does this wavelength belong ?