The given figure depicts the atomic structure of an atom of an element ‘X’.
Write the following information about the element ‘X’.
- Atomic number of ‘X’
- Atomic mass of ‘X’
- Valence electrons
- Valency of ‘X’
- ‘X’ should be metal or non-metal.
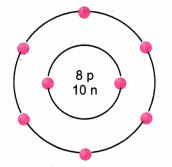
Answer:
- Atomic number = Number of protons = 8
- Atomic mass = Number of protons + Number of neutrons = 8 + 10 = 18 u
- Valence electrons = 6
- Valency of ‘X’ = 8 - 6 = 2
- ‘X’ should be non-metal because there are six valence electrons hence it will take two more electrons to complete its outermost shell.