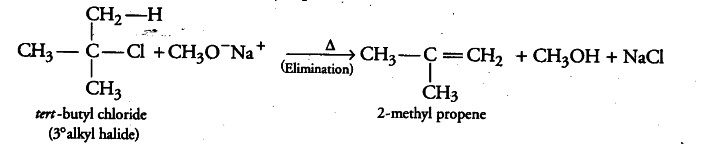The following is not an appropriate reaction for the preparation of tert-butyl methyl ether:
![]()
(i) What would be the major product of the given reaction?
(ii) Write a suitable reaction for the preparation of tert-butyl methyl ether, specifying the names of reagents used. Justify your answer in both cases.
(i) As the given alkyl halide is tertiary in nature, therefore on reaction with sodium methoxide, elimination reaction takes place in place of substitution and hence, alkene is formed as a major product. Therefore, in the given reaction, 2-methyl propene is formed as a major product
(ii) For the preparation of tert-butyl methyl ether, i.e. an unsymmetrical ether, the alkyl halide should be primary because the reaction between alkyl halide and alkoxides follows mechanism and primary alkyl halides are most reactive towards mechanism. Thus, for the preparation of terr-butyl methyl ether, methyl chloride should react with sodium tert-butoxide.