The first ({{\vartriangle }_{i}}{{H}_{1}}) and the second ({{\vartriangle }_{i}}{{H}_{2}}) ionization enthalpies (in kj {{mol}^{-1}}) and the ({{\vartriangle }_{eg}}{{H}_{1}}) electron gain enthalpy (in kj {{mol}^{-1}}) of a few elements are given below:
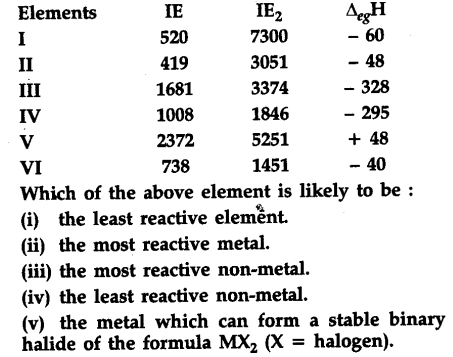
The least reactive element is V. It is Helium. It has no tendency to lose electrons [as is clear from its high I${{E}{1}}$ and its higher I${{E}{2}}$ nor can it gain electrons [positive value of electron gain enthalpy - energy has to be absorbed to gain or add an electron].
(ii) The most reactive metal is II. It is K. Its I${{E}{2}}$ is lowest and I${{E}{2}}$ is very high, i.e., it can lose one electron readily from its valence shell [4th] to become ${{K}^{+}}$. It cannot lose 2nd electron readily because of stable Ar core.
(iii) The most reactive non-metal is III It is fluorine,
whose I${{E}{1}}$ and I${{E}{2}}$ are very high, so it cannot lose
electrons readily. On the other hand, its electron
gain enthalpy has a large negative value indicating
it can accept an electron readily.
(iv) The least reactive non-metal is IV It is iodine.
Its I${{E}{1}}$ and I${{E}{2}}$ are very high, meaning it cannot lose
electrons readily. On the other hand, its electron
gain enthalpy has a large negative value (but not
as large negative value as F above) indicating that
it can accept an electron readily.
(v) The metal which can form a stable binary
halide of the formula M${{X}{2}}$ (X = halogen) is VI.
It is magnesium Mg + ${{X}{2}}$----> Mg${{X}{2}}$
Its I${{E}{1}}$ and I${{E}{2}}$ are not very high but its electron
gain enthalpy is very small,I${{E}{2}}$ is not very large
as compared to I${{E}{1}}$.
Because of its electropositive nature and tendency
to lose two electrons, it can form a stable binary
halide M${{X}{2}}$.