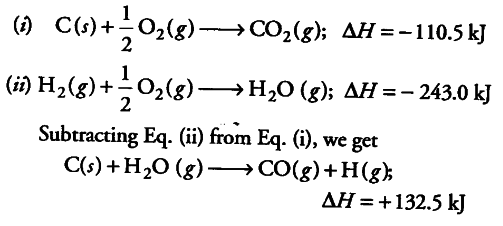The enthalpy of formation of carbon monoxide and steam are -110.5 and -243.0 kJ respectively. Calculate the heat of the reaction when steam is passed over coke as
C+ ${ H }{ 2 }O$ ------->
CO + ${ H }{ 2 }$
We are given
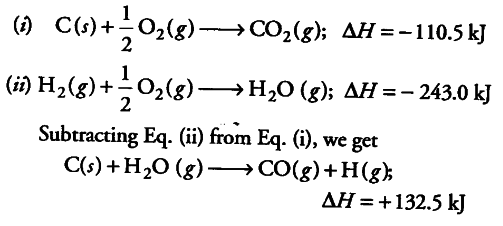
The enthalpy of formation of carbon monoxide and steam are -110.5 and -243.0 kJ respectively. Calculate the heat of the reaction when steam is passed over coke as
C+ ${ H }{ 2 }O$ ------->
CO + ${ H }{ 2 }$
We are given