The electrons in the atoms of four elements A, B, C and D are distributed in three shells having, 3, 5 and 7 electrons in the outermost shell respectively. State the period in which these elements can be placed in the modern periodic table. Write the electronic configuration of the atoms of A and D and the molecular formula of the compound formed when A and D combine.
| Elements | Valence electrons | Period |
|---|---|---|
| A | 1 | 3 |
| B | 3 | 3 |
| C | 5 | 3 |
| D | 7 | 3 |
Electronic configuration of A: Is2 2s2 2p6 3s1
Electronic configuration of D: Is2 2s2 2p6 3s2 3p5
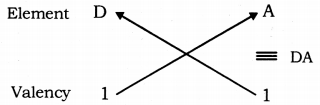
Molecular formula of the compound formed with A and D:
Atomic number of A = 11
Electronic configuration A = 2, 8, 1
Number of valence electrons of A = 1
Valency of A = 1
Atomic number of D = 17
Electronic configuration D = 2, 8, 7
Number of valence electrons of D = 7
Valency of D = 8 - 7 = 1