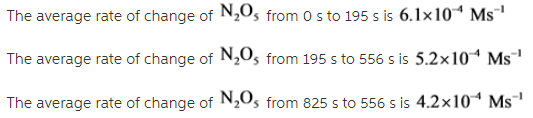The decomposition of N2O5 can be described by the equation.
2N2O5 (soln) —> 4NO2 (soln) + 2 (g)
Given this data for the reaction at 45 degrees C in carbon tetrachloride solution, calculate the average rate for each successive time interval.
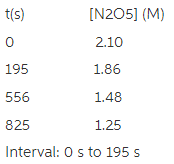
Reaction rate= _____M/s
195 s to 556 s
Reaction rate= _____M/s
556 s to 825 s
Reaction rate= _____M/s
Answer: