The crystal AB (rock salt structure) has molecular weight 6.023 y u where, y is an arbitrary number and u is the unified atomic mass unit. If the minimum distance between cation and anion is and the observed density is 20 kg/${{m}^{3}}$ then find the
(i) density in kg / ${{m}^{3}}$ and
(ii) type of defect.
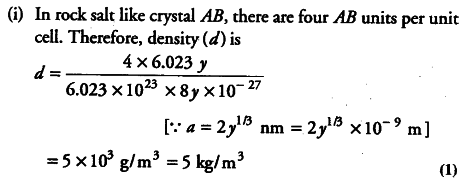
(ii) Since, observed density is greater than expected, theoretical
density, so there must be some excess metal occupying interstitial spaces. This type of defect is known as metal excess defect