The atomic radii of the element of second period are given below:
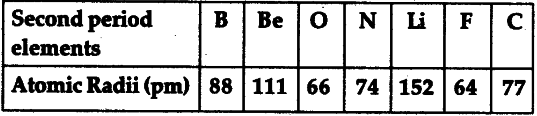
(i) Arrange these elements in decreasing order of their atomic radii.
(ii) Are the elements now arranged in the pattern of a period in the periodic table ?
(iii) Name the element which has the (a) largest and (b) smallest atomic number.
(iv) From the above data, infer how the atomic size or atomic radius of the elements changes as we go from left to right in a period.
(v) Name one metal, one non-metal and a metalloid out of these elements.
(vi) Why does atomic radius decreases as we move from left to right in a period?