State as to why
(a) alkali metals are prepared by electrolysis of their fused chlorides?
(b) sodium is found to be more useful than potassium?
(ii) Explain what happens when fused sodium metal reacts with ammonia?
(i) (a) I. Alkali metals are strong reducing agents, hence cannot be extracted by reduction of their oxides and other compounds.
II. Being highly positive in nature it is not possible to displace them from their salt solutions by any other element.
III. Alkali metals cannot be obtained by the electrolysis of the aqueous solution of their salts because { H }_{ 2 } is liberated at cathode instead of alkali metal. That’s why alkali metals are prepared by electrolysis of their fused chloride,
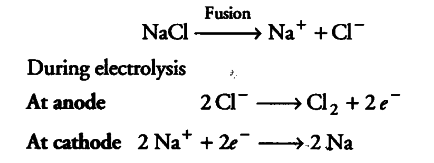
(b) Sodium is found to be more useful than potassium as it is highly reactive but not as reactive as potassium. Sodium is used
I. as a coolant in nuclear reactor.
II. in the manufacture of tetraethyl lead an anti-knock additive for petrol.
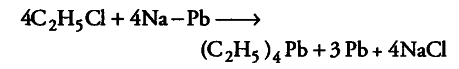
III. In sodium vapour discharge lamps.
IV. As a laboratory reagent for organic analysis.
![]()