Sodium metal from sodium chloride : Sodium is prepared from fused (molten) sodium chloride. Sodium chloride is mixed with Ca$C{{l}_{2}}$ and KF [to lower the M. Pt. of NaCl to 850-875K] and subjected to electrolysis (in DOWN’S CELL) when the following reactions occur:
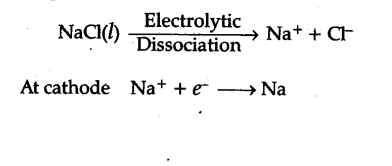
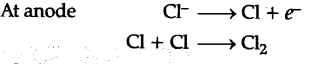
Sodium, liberated at the cathode, is collected in kerosene oil, chlorine gas is liberated at the anode.
(ii) Sodium hydroxide from sodium chloride : Sodium hydroxide (caustic soda) is generally pre-pared by the electrolysis of brine solution (NaCl solution in water) in Castner Kellner cell. A mercury cathode and carbon anode are used, Sodium metal discharged at the cathode combines with mercury to form sodium amalgam. C{{l}_{2}} gas is evolved at the anode.
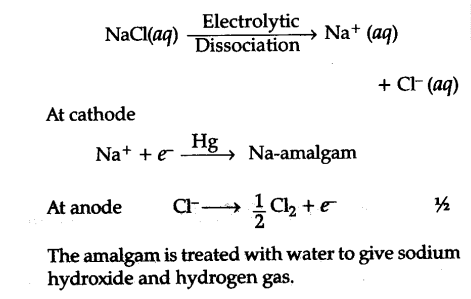
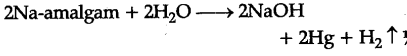
(iii) Sodium carbonate (${{Na}{2}}C{{O}{3}}) from sodium
chloride : Sodium carbonate is prepared from an
aqueous solution of NaCl by Solvay Process. In
this process C{{O}_{2}}$ is passed through NaCl solution
saturated with ammonia, when following reactions
occur.
