Specific Heat, J/g.°C
2.06 - ice
4.18 - water
2.03 - steam
Molar heat of fusion for water, kJ/mol - 6.02
Molar hear of vaporization for water, kJ/mol - 40.6
Use the information given above, calculate the energy involved in the conversion of 235 g of steam at 373 K to ice at 243 K. Be sure to use the correct sign and answer in kJ.
Concepts and reason
Specific heat is the energy required to raise the temperature of 1 g of a substance by![]() .
.
To calculate the energy released to change the temperature, use the formula,
Answer:

Explanation:
Mass of the steam is given as 235 g. The specific heat for steam is ![]() . These values are substituted and heat released is calculated.
. These values are substituted and heat released is calculated.
Take the temperature in C units. To convert the temperature from K to C , subtract 273 from it.
First, calculate the moles of steam as follows;

During the phase change, the change in temperature is zero. In this case, use the following formula to calculate the heat required:
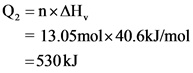
Explanation:
Here, the phase changes from steam to liquid. Hence, here molar heat of vaporization should be used.
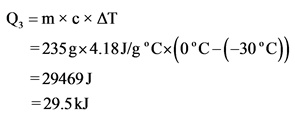
Explanation:
Here, the temperature of water changes. Hence, the specific heat of water should be substituted for c.

Explanation:
Here, the phase changes from water to ice. Hence, the heat of fusion should be used here.
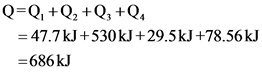
Here, heat is released during the process. Hence, the heat energy released is ![]() .
.

Explanation:
The energy is released during the process of conversion of steam at 373 K to ice at 243 K. Hence, the sign of the energy involved in the process is negative.
