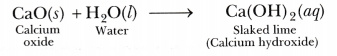
Solid calcium oxide was taken in a container and water was added slowly to it.
(i) State the two observations made in the experiment.
(ii) Write the name and chemical formula of the product formed.
Answer:
Following are the two observations:
(i) Calcium oxide (CaO) reacts vigorously with water to form slaked lime.
The container becomes hot because a large amount of heat is released during this reaction.
(ii) The product formed is slaked lime for which the chemical formula is Ca(OH)2