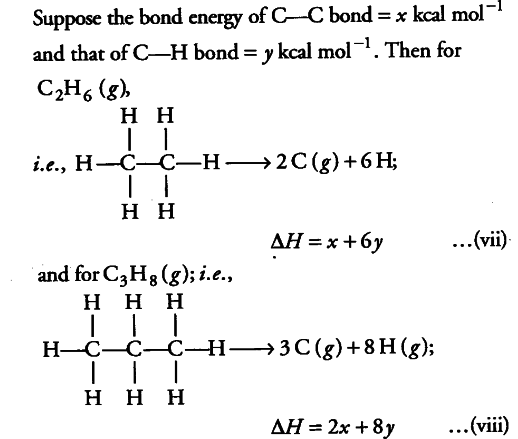
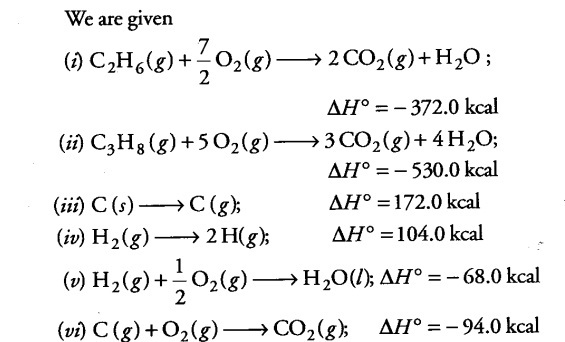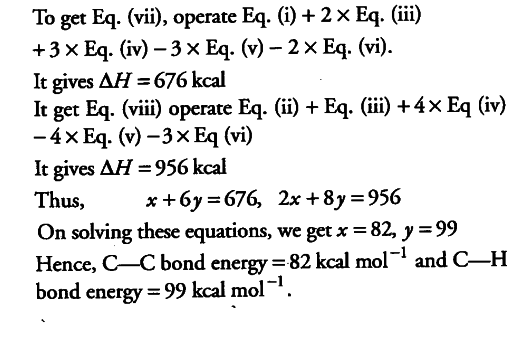Using the data (all values are in kilocalories per mole at 25° C) given below, calculate the bond energy of C—C and C—H bonds.
∆H° combustion (ethane) = -372.0
∆H° combustion(propane) = -530.0
∆H°for C (graphite) -----> C(g) = 172.0
∆fH° of $H { 2 }O$ (l) = -68.0
∆H° of ${{CO}{2}}$ (g) = -94.0