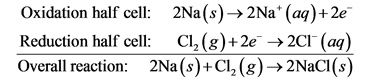Separate this redox reaction into its balanced component half-reactions.
Cl2+2Na---->2NaCl
Oxidation of half reaction:
Recuction half reaction:
Concepts and reason
Cell potential is the potential difference between the two half cells in an electrochemical cell. The potential difference is created by the movement of electrons from one half cell to another.
The given electrochemical cell contains two half cells which adds up to give the overall balanced reaction.
Fundamentals
An electrochemical cell consists of two half cells. In one half, oxidation (loss of electron) occurs and in other half, reduction (gain of electron) occurs.
The oxidation half-cell is termed as anode and the reduction half-cell is termed as cathode.
Answer:
At anode, oxidation occurs
![]()
At anode of the electrochemical cell, oxidation occurs. That is, there is a loss of electron.
At cathode, reduction occurs,
![]()
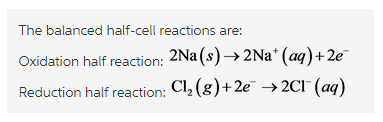
Explanation:
At cathode of the electrochemical cell, reduction occurs. That is, there is a gain of electron. And the final overall equation comes after adding these two equations, that is,