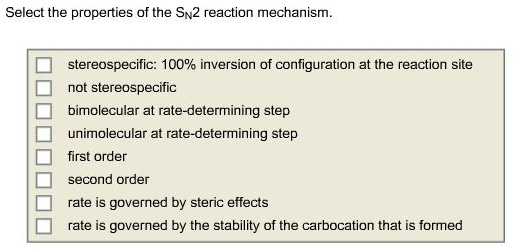
Select the properties of the Sn2 reaction mechanism.
Concepts and reason
SN2 reaction is a nucleophilic substitution reaction. Here, the number 2 indicates bimolecular. The nucleophile attacks from the opposite side of the leaving group. Hence, it gives inversion of configuration.
Fundamentals
SN2 reaction is a single step reaction. The rate of the reaction depends on both concentration of substrate and concentration of nucleophile. SN1 reaction is a two-step reaction. The rate of the reaction in SN1 depends only on the concentration of substrate.
Molecularity is the number of molecules that are present in a rate determining step. That is the sum of the stoichiometric coefficients of the reactants in the rate determining step.
Answer:
The rate of the reaction depends on both concentration of substrate and concentration of nucleophile. Hence, it is second order reaction and its molecularity is 2.
If the rate of the reaction depends only on the concentration of substrate, it is first order reaction. If the rate of the reaction depends on both concentration of substrate and the concentration of nucleophile, it is a second order reaction.
In SN2 reaction mechanism, as the bond between leaving group and the substrate weakens, the bond between the incoming nucleophile and the substrate forms. Hence, the rate of the reaction decreases, as the steric hindrance increases. Therefore, rate is governed by steric effects.
The nucleophile attacks from the opposite side of the leaving group. Hence, 100% inversion of configuration takes place at the reaction site. That means it is stereospecific.
Hence, the correct options are as follows:
- stereospecific: 100% inversion of configuration at the reaction site
- bimolecular at rate-determining step
- second order
- rate is governed by steric effects
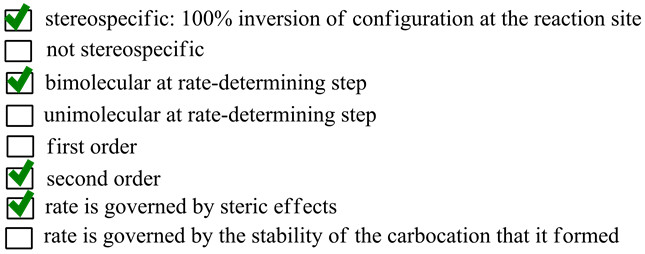
SN2 reaction occurs in a single step. The breaking of bond between carbon and the leaving group and formation of bond between carbon and attacking nucleophile occurs simultaneously. Hence, no carbocation is formed in SN2 reaction mechanism. Thus, the option ‘rate is governed by the stability of the carbocation that is formed’ is wrong.