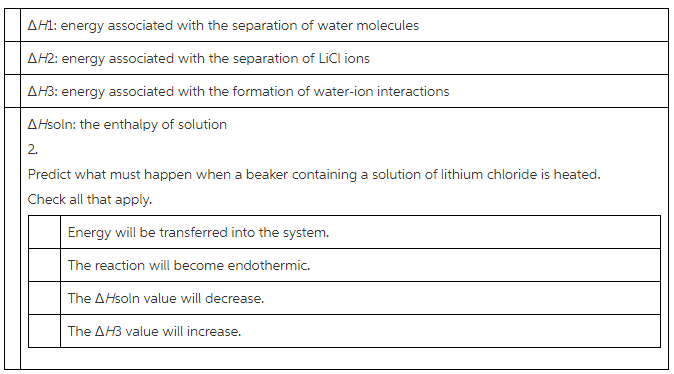
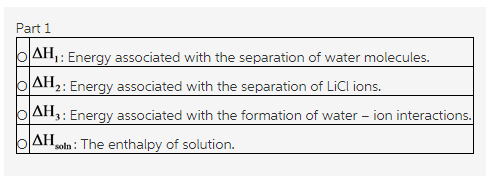
1.Select the ΔH values associated with the dissolution of lithium chloride that are exothermic.
Check all that apply.
Concepts and reason
The change in heat content of the system is represented as Enthalpy. It is a thermodynamic quantity and is denoted as ![]() .
.
Fundamentals
If the system absorbs the heat, then it is represented as endothermic.
If the system releases the heat, then it is represented as exothermic.
The change in enthalpy of the system depends upon the type of reactions present.
Answer:
(1)
![]() : Energy associated with the separation of water molecules.
: Energy associated with the separation of water molecules.
Water molecules separated into ions. The separation of ions requires energy. Therefore, it is the endothermic process.
![]() : Energy associated with the separation of LiCl ions.
: Energy associated with the separation of LiCl ions.
To separate LiCl to ions require energy. Therefore, it is the endothermic process.
![]() : Energy associated with the formation of water – ion interactions.
: Energy associated with the formation of water – ion interactions.
Water – ion interactions release energy. Therefore, it is the exothermic reaction.
Explanation:
When the water molecules come in contact with the ions, they release energy. The release of energy is represented as exothermic process.
![]() : The enthalpy of solution.
: The enthalpy of solution.
Explanation:
When Lithium chloride (LiCl) is dissolved in water, it releases heat. The release of heat is represented as exothermic process.