Salt A commonly used in bakery products on heating gets converted into another salt B which itself is used for removal of hardness of water and a gas C is evolved. The gas C, when passed through lime water turns it milky. Identify A, B and C.
Salt A is sodium hydrogen carbonate (NaHCO3).
When sodium hydrogen carbonate is heated, it decomposes to give sodium carbonate (Na2CO3), water and carbon dioxide
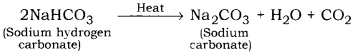
Hence, salt ‘B’ is sodium carbonate and the gas evolved ‘C’ is carbon dioxide. We know that, C02 when passed through lime water turns milky.