Rank these systems in order of decreasing entropy.
Concepts and reason
Entropy
Entropy is defined as the measure of disorder (randomness) of molecules such as helium, chlorine, and hydrogen peroxide in a system. It is a thermodynamic state function.
Fundamentals
Entropy
The entropy of molecules increases with the changing of the physical states of matter from solid to gas due to internal degrees of freedom of motions.
![]()
The entropy of molecules tends to increase with the increase of the following factors:
oTemperature (T)

Answer:
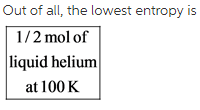
The ½ mol of liquid helium at 100 K is in liquid physical state. Therefore, this system has the lowest entropy than other.
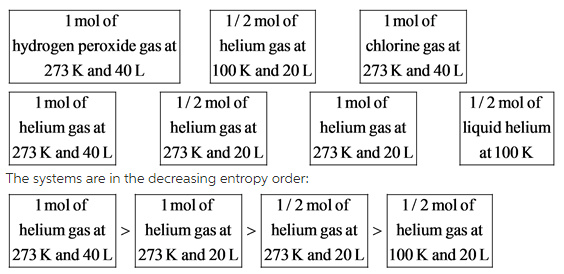
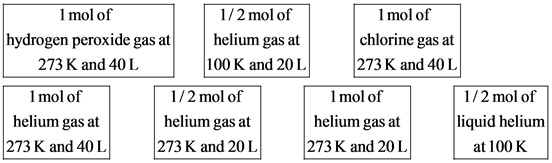
The ½ mol of helium gas at different T and V are less entropy than that of 1 mol of helium gas.
At the same volume (20 L), the ½ mol of helium gas at 273 K system is greater entropy than the ½ mol of helium gas at 100 K system.
At the same temperature (273 K), the 1 mol of helium gas at 40 L system is higher entropy than the 1 mol of helium gas at 20 L system.
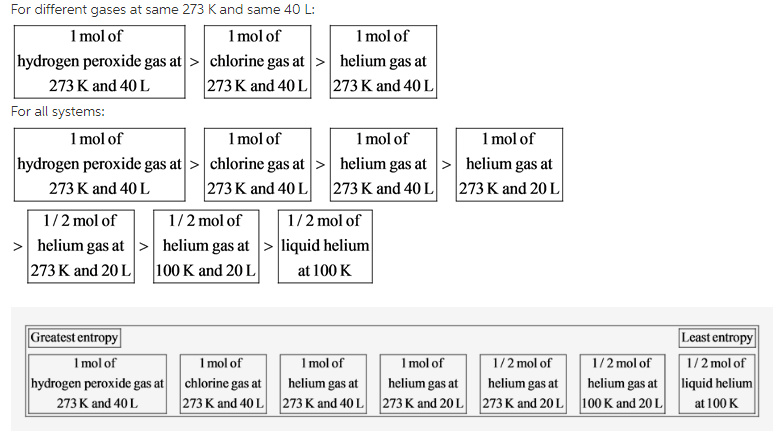
The number of atoms of helium, chlorine, and hydrogen peroxide molecules are 1, 2, and 4, respectively. Therefore, the larger gas molecule in the given systems is hydrogen peroxide.
Hydrogen peroxide, ![]() gas molecule, contains two hydrogen atoms and two oxygen atoms while chlorine,
gas molecule, contains two hydrogen atoms and two oxygen atoms while chlorine, ![]() gas molecule, contains two chlorine atoms only. Therefore, the 1 mol of hydrogen peroxide gas at 273 K and 40 L system has greater entropy than the 1 mol of chlorine gas under the same conditions.
gas molecule, contains two chlorine atoms only. Therefore, the 1 mol of hydrogen peroxide gas at 273 K and 40 L system has greater entropy than the 1 mol of chlorine gas under the same conditions.
Helium, He gas molecule, contains only one helium atom while chlorine, ![]() gas molecule, contains two chlorine atoms only. Therefore, the 1 mol of helium gas at 273 K and 40 L system has lesser entropy than the 1 mol of chlorine gas under the same conditions. Therefore, the 1 mol of chlorine gas at 273 K and 40 L system is placed after the 1 mol of hydrogen peroxide gas at 273 K and 40 L system and before the 1 mol of helium gas at 273 K and 40 L system.
gas molecule, contains two chlorine atoms only. Therefore, the 1 mol of helium gas at 273 K and 40 L system has lesser entropy than the 1 mol of chlorine gas under the same conditions. Therefore, the 1 mol of chlorine gas at 273 K and 40 L system is placed after the 1 mol of hydrogen peroxide gas at 273 K and 40 L system and before the 1 mol of helium gas at 273 K and 40 L system.