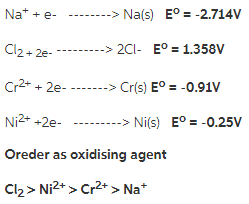Rank these species by their ability to act as an oxidizing agent.
Na+, Cl2, Cr^2+, Ni^2+
Answer:
To solve this question we must look their position in standard electrode potential table. The greater he standard electrode potential the greater will be the ability to act as oxidising agent.