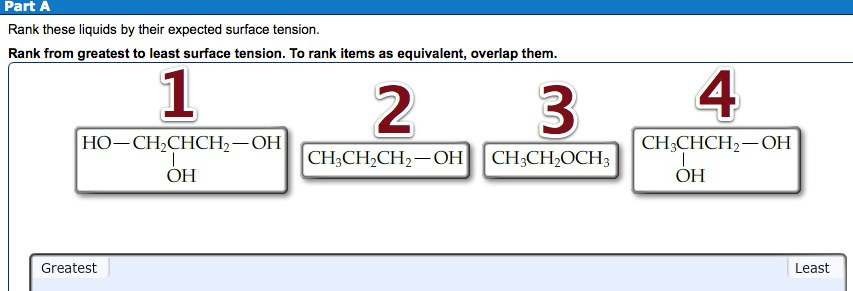
Rank these liquids by their expected surface tension. Rank from greatest to least surface tension.
Concepts and reason
Determine the type of liquids in the list and categorize them based on the forces present in them.
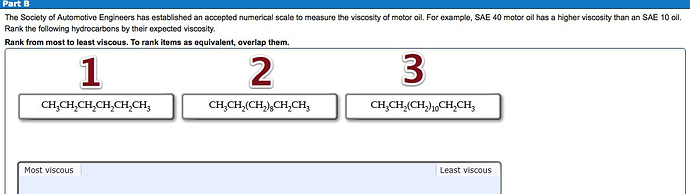
Check the surface occupied by the given compounds and put them in order of their viscosities.
Fundamentals
Surface tension is defined as the forces between the molecules which keep the compound together. It makes the liquid to stay together. The property of surface area is dependent on the forces present in the liquid.
Hydrogen bonding present in the organic molecule due to the presence of hydroxyl group affects the surface area of the liquid. The higher the number of hydroxyl groups, the higher the surface tension.
The compound having larger surface area have high intermolecular forces. Therefore, the viscosity is higher for a molecule with higher intermolecular forces.
Answer:
(A)
The order of surface tension of the given class of compounds is as follows:
Alcohols > Ethers
Alcohols form hydrogen bonding with other molecules, which increases the surface area of the molecule.
Ethers form much less hydrogen bonding as compared to alcohols, therefore, they have less surface area than alcohols.
The order of surface tension from greatest to least is as follows:

The compound 1 has three hydroxyl groups, compound 4 has two hydroxyl groups and compound 2 has one hydroxyl groups. More the hydroxyl group, more will be the hydrogen bonding, which results in greater surface tension.
(B)
The order of viscosity from most to least is as follows:
![]()
Compound 3 occupies large surface area compound to the compound 2 and 1. This is because compound 3 has the longest carbon chain, followed by compound 2 and 1.