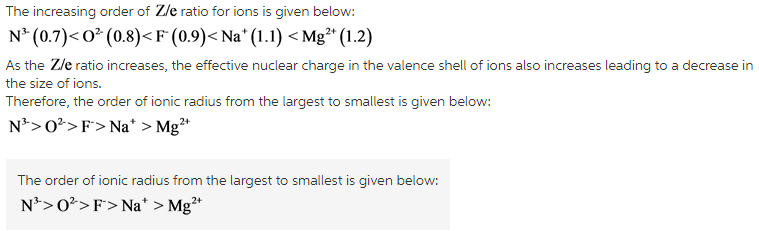Rank these ions according to ionic radius from largest to smallest:
Na+ Mg2+ N3- O2- F-
Concepts and reason
Ions are formed when neutral atom accepts or donates the electrons. Ionic radius may be smaller or larger depending on the charge present in it. Ionic radius is the distance between the nucleus and valence shell of an ion. For ions with the isoelectronic series (same number of electrons and different number of protons), the order of ionic radii can be identified by finding the ![]() ratio.
ratio.
Fundamentals
Isoelectronic series: The ions having the equal number of electrons, the size depends on ![]() ratio. The term “Z” is atomic number and “e” is number of electrons.
ratio. The term “Z” is atomic number and “e” is number of electrons.
oAs the ![]() ratio increases, the ionic radius decreases due to a greater nuclear force on the valence shell.
ratio increases, the ionic radius decreases due to a greater nuclear force on the valence shell.
oAs the ![]() ratio decreases, the ionic radius increases due to a lesser nuclear force on the valence shell.
ratio decreases, the ionic radius increases due to a lesser nuclear force on the valence shell.
Answer:
The ions are given below:
![]()

Write the atomic number and number of electrons present in each ion

The atomic number for each of the given atoms is identified from the periodic table. Note that all the ions have equal number of electrons equal to 10.