Rank these elements according to electron affinity from most energy released by gaining an electron to most energy absorbed by gaining an electron …Si ,Kr ,Cl.
Concepts and reason
Electron affinity is the one form of energy that is released when the neutral atom in a gaseous state converts to the negatively charged ion by taking an extra electron. Completely filled and half-filled elements have higher electron affinity values.
Electron affinity of various elements depends on:
- Size of atom
- Electronic configuration
- Screening effect
Fundamentals
The electron affinity for an arbitrary element is depicted below:
![]()
If the magnitude of electron affinity is negative, an atom or a molecule gains an electron and if the magnitude of electron affinity is positive, an atom or a molecule is forced to gain an electron.
Periodic trends for electron affinity are given below:
oAlong the periods, moving from left to right, the magnitude of electron affinity increases.
oAlong the groups, moving from top to bottom, the magnitude of electron affinity decreases
Factors affecting electron affinity values are given below:
oSize of atom: As the size of an atom increases, the value of electron affinity decreases due to the decrease of effective nuclear charge on valence electrons.
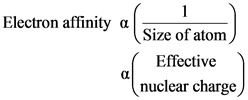
oScreening effect: As the screening effect increases, the electron affinity decreases. Due to the increase of number of electrons, the shielding effect between the nucleus and valence electrons increase.
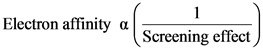
oElectronic configuration: The elements with fully-filled and half-filled configuration have extra stability. Due to this, high energy is required to add the electron to such elements.
Answer:
The elements that can release energy by gaining an electron are “Chlorine and Silicon”.
![]()
Explanation:
On moving from left to right across the periodic table, electron affinity increases. Therefore, chlorine has a higher electron affinity than silicon. The magnitude of electron affinity of chlorine is negative, which indicates that more energy is released by gaining an electron and a silicon atom releases relatively less energy than chlorine by gaining an electron.
The element that can absorb energy by gaining an electron is “Krypton”.
The electron affinity of krypton is ![]()
The order of elements according to electron affinity from most energy released by gaining an electron to most energy absorbed by gaining an electron is given below:
![]()
Explanation:
Krypton is a noble gas and it has zero electron affinity value due to the presence of completely filled electron configuration. There are no vacant orbitals to place an extra electron in krypton. The size of a chlorine atom is relatively lower than silicon, since the size of an atom is inversely proportional to electron affinity; the electron affinity of chlorine is higher than silicon, followed by krypton.
