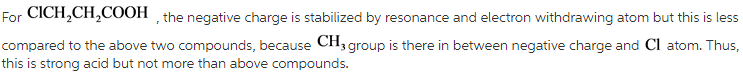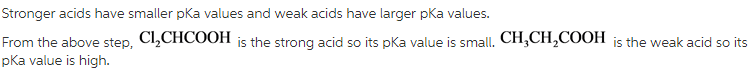Rank these acids according to their expected pKa values.
ClCH2COOH
ClCH2CH2COOH
CH3CH2COOH
Cl2CHCOOH
In order of highest pka to lowest pka
Concepts and reason
PKa is the negative logarithm of dissociation constant. It gives the relative strengths of the acids. Stronger acids have smaller pKa values and weak acids have larger pKa value.
Fundamentals
The stronger the acid, the weaker its conjugate base. The larger the pKa of the conjugate base, the stronger the acid. The strength of an acid is inversely related to the strength of its conjugate.
Answer:
(1)
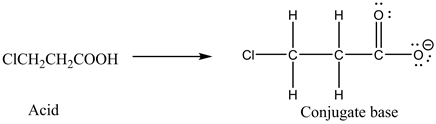
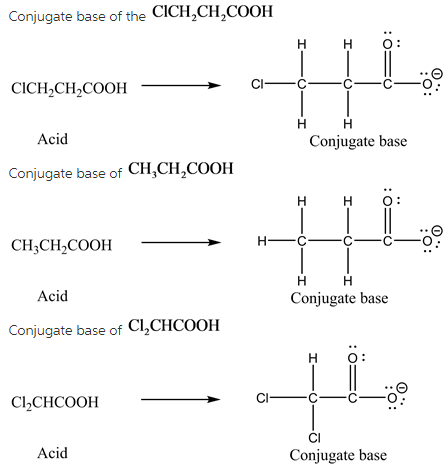
Conjugate base of ![]()
Explanation:
Conjugate base is the substance which has one less proton than the parent acid.
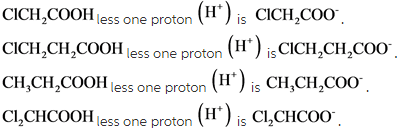
(2)
![]()
Explanation:
![]()
(3)
The order of highest pKa to lowest pKa is,
Explanation: