PART A:
Arrange the elements in decreasing order of first ionization energy.
Rank from highest to lowest first ionization energy. To rank items as equivalent, overlap them.
In, Ge, Se, Cs
PART B:
Arrange the elements in order of decreasing first ionization energy. Rank from highest to lowest first ionization energy. To rank items as equivalent, overlap them.
element x (radius (pm): 110)
element y (radius (pm): 199)
element z (radius (pm): 257)
Concepts and reason
Ionization energy:
It is the energy required to remove an electron from a neutral atom in the gaseous phase.
First ionization energy:
First ionization energy is also called as initial ionization energy,![]() of an atom or molecule is the energy required to remove one mole of electron from one mole of isolated gaseous atoms or ions.
of an atom or molecule is the energy required to remove one mole of electron from one mole of isolated gaseous atoms or ions.
Fundamentals
Ionization energy and periodic table:
1.From left to right, the valance shell stability increases and hence, ionization energy of the elements increases.
Noble gases have high ionization energy as their valance shells are fully occupied.
2.From top to bottom, the electron shielding effect increases and hence, ionization energy of the elements decrease.
As the size increases, ionization energy decreases.
Answer:
Part A
The decreasing order of ionization energy is as follows:
![]()

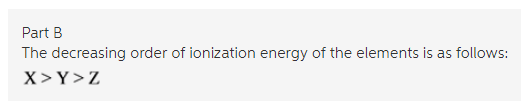
Part B
The decreasing order of ionization energy is as follows:
![]()